Technology
What is Graphene
WHAT IS GRAPHENE?
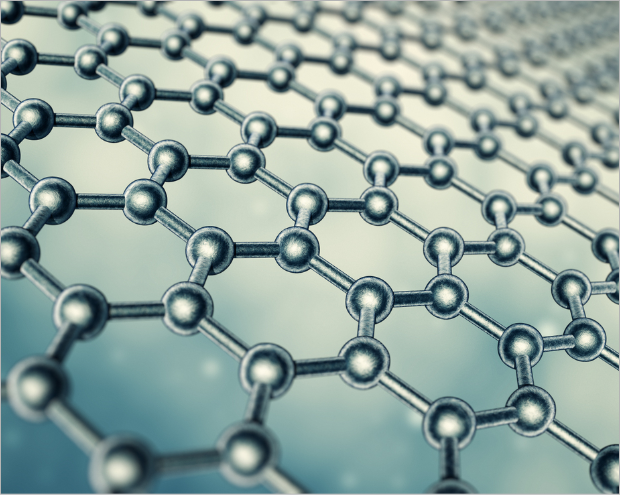
Graphene is a form of carbon allotrope consisting of planar sheets which are one atom thick carbon atoms arranged in a honeycomb-shaped (hexagonal) lattice. Graphene is considered as an attractive material in many industrial fields due to its unique and diverse excellent properties. It is about 100~300 times stronger than steel with tensile stiffness ~1020 GPa. It is the best conductor of heat at room temperature (thermal conductivity ~ 5300 W·m−1·K−1) and also the best conductor of electricity known (studies have shown electron mobility at values of more than 15,000 cm2·V−1·s−1) and it is optically transparent (with light absorption at πα ≈ 2.3% of white light or 97.7 % transmittance). Because it is less than one atomic thick (0.33 nm), or about one million times thinner than a sheet of paper, it is a very light material weighing about 0.77 mg/m2 and has an extremely high surface area (theoretical specific surface is of 2630 m2/g). Graphene is the subject of constant research and is believed to revolutionize the entire industry as it has many potential properties of graphene-based materials and applications.
HISTORY OF GRAPHENE
The term graphene was introduced in 1986 by chemists Hanns-Peter Boehm, Ralph Setton and Eberhard Stumpp. It is a combination of the word graphite and the suffix -ene, referring to polycyclic aromatic hydrocarbons. Discovered in 2004 by Prof. Geim and Novoselov Awarded Nobel Prize in Physics in 2010 for “groundbreaking experiments regarding the two-dimensional material graphene”. Geim and Novoselov used the “Scotch tape” method to extract graphene from graphite to obtain a one atom-thick piece of graphene. Today’s graphene is manufactured by various synthetic methods such as CVD, epitaxial growth, and hummer’s method, and etc. for mass production and large area production.
TYPE OF GRAPHENE

Graphite (3D)
Carbon exists in the nature
Graphite has a layered structure consisting of rings of six carbon atoms arranged in a horizontal sheet with wide spacing. Bonding between layers is via weak van der Waals bonds, which allow layers of graphite to be easily separated, or to slide past each other.
Cnhemical vapor deposition, Epitaxial growth & Hummer’s method (for graphee oxide)
Carbon exists in the nature
Graphene and graphene oxide are a form of carbon allotrope composed of planar sheets, one atom-thick carbon atoms arranged in a honeycomb (hexagonal) lattice. It can exist as a single-layer or multi-layered structure by controlling the method of synthesis.

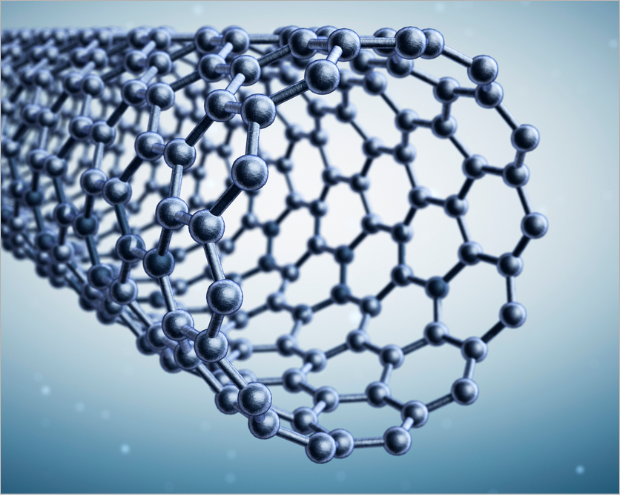
Carbon Nanotube (1D)
Vapor Phase Growth, High Pressure Carbon Mnoxide
Carbon nanotubes (CNTs) are graphene sheets rolled into a cylindrical shape. The diameter of the cylinder shape is a few to tens of nanometers, so it is called a carbon nanotube.
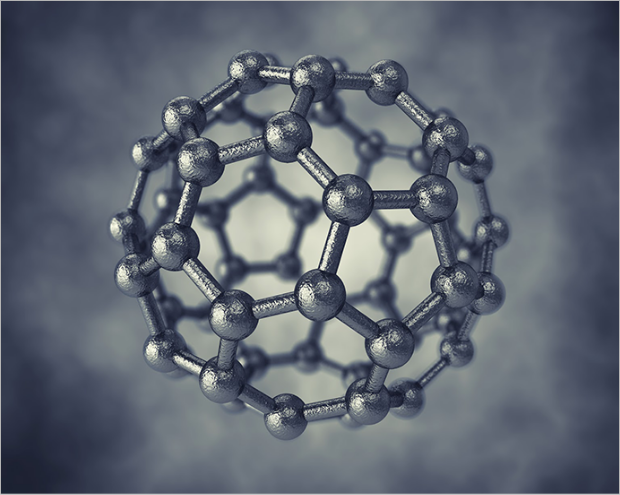
Fullerene (0D)
Arc-discharger
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected so as to form a closed or partially closed mesh. Molecules can be hollow spheres, ellipsoids, tubes, or many other shapes and sizes.
WHAT IS CARBON?